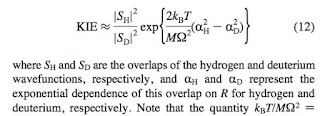An important but basic research skill: not getting too distracted
Making progress in science requires significant focus and discipline. In some sense you need to put in your 10,000 hours. People can distracted by all sorts of non-scientific pursuits: Facebook, romance, family dramas, hobbies, partying, .... But this post is about scientific distractions. I illustrate this will two extreme caricatures. John really wants to understand his Ph.D project in experimental solid state physics at a deep level. He thinks the quantum measurement problem is really interesting and so he is reading a lot of papers about that. The software he needs for his experiment is functional but he does not like some of the way it interfaces with other software and so he is rewriting it all. In one month he is giving a talk at a conference and so he is not going in the lab for the next month because he wants to give a really nice talk. Whenever his advisor gives him a paper to look at he not only reads it but some of the background references. He spends a lot of time talki