Another example of competing quantum effects in hydrogen bonds

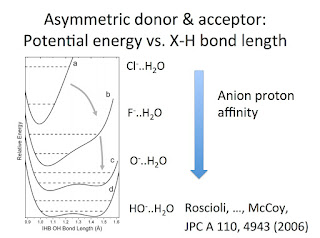
Previously, I have highlighted how one of the organising principles for understanding quantum nuclear effects in hydrogen bonding is that of competing quantum effects. This idea features in this talk and this recent review about water. Basically, as the strength of the hydrogen bond in an X-H...Y systems increases, the zero point energy associated with the X-H stretch (bending) vibrational modes increases (decreases). The effect manifests in a wide range of isotope effects where hydrogen is replaced with deuterium. The relative magnitude of these competing effects changes with the bond strength, and so the sign of the isotope effects can be positive or negative. This week I learned of another nice example of competing quantum effects in the paper. Why Does Argon Bind to Deuterium? Isotope Effects and Structures of Ar·H 5O 2 + Complexes Laura R. McCunn, Joseph R. Roscioli, Ben M. Elliott, Mark A. Johnson, and Anne B. McCoy The figure below shows the ground state geometry